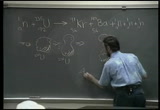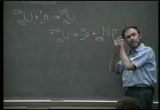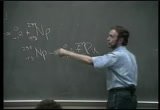tv Democracy Now LINKTV April 24, 2013 8:00am-9:00am PDT
8:00 am
8:01 am
from one kind to another. and one of the reactions that did succeed was kinda novel. instead of throwing higher and higher velocity particles at matter, three people in germany, lisa meitner, otto hahn, otto-- strassman, i think, anyway, what they did was they threw slow moving neutrons at uranium isotopes and they found a reaction that changed the world. and the reaction that changed the world is that what you see before you here. it's in your textbooks. the first page in the chapter, fission and fusion. and all we're saying is that it turns out if a slow moving neutron taps into uranium 235 isotope-- [makes sounds] --it will lay it right in half, and the isotopes, instead of a little particle coming off like maybe a proton or an alpha particle, the whole things falls on half. wild. and-- [makes sounds] here's a typical reaction. there are many reactions. this is just the typical one. the uranium busts in half into krypton and barium. these are about halfway down the periodic table from where the uranium is.
8:02 am
and the neat thing was, is there were also an ejection in the average of about three more neutrons. now, if you can slow these neutrons down-- they're going kinda fast-- if you can slow those down so they going slow enough to cause another reaction, then you have a chain reaction. and you know what you do? you throw these neutrons into other atoms. hey, let me ask you a physics question. if you wanna throw a ball into some other balls, such that the ball will slow down, would you throw the ball into an environment of heavy balls or light balls? heavy balls. and you want the thing to slow down. let me ask you this. if i take a ball and i throw it against a brick wall-- [makes sounds] --when it bounces off, will it bounce off a lot slower or about the same? about the same. about the same. if i take a golf ball and i throw it against a bowling ball, is that bowling ball gonna slow the golf ball down? no, it's not. so what you do is you take the golf ball and you throw into an arena of ping-pong balls. and if you do that, it will give what? give the energy to ping-pong ball
8:03 am
and it itself will slow down. and so what do you do is you take these things and you bounce them off atoms like the size of carbon. did you ever hear about the heavy water? you bounce them off light atoms or molecules and these things will slow down, so they're moderated and will cause the reaction of more of this fission. you call--nuclear fission, gang, breaking apart, nuclear fission, and you will fission more atoms. anyway, this is something that cause an awful lot of excitement. because along with these two, it turns out the kinetic energy of these particles and these, all flying apart, is awesome. the energy that takes to light up new york city comes about as a result of water pouring over niagara falls. and every water drop has an energy of about this much, four electron volts. electron volts are tiny unit of energy. it's microscopic unit of energy, yeah? but four electron volts per water drop, tnt-- [makes sounds] --you get about 30 electron volts. high-octane gasoline,
8:04 am
about 30 electron volts per molecule of combustion, yeah? one atom, u235, fissions, you get about 200 million electron volts of energy. awesome, awesome. an awful lot of energy for one atom, and that kinda changed the world. and so we now talk about the atomic age or more properly the nuclear age because we're talking about an awful lot of energy for just a little bit of matter. it turns out the most common isotopic uranium is uranium-- let me try it over here-- uranium 238. and when 238 catches a neutron, what it does is it turns to u239. u238 emits alpha particles, but 239 emits beta particles.
8:05 am
and guess what the 239 does, gang? it turns into an element beyond uranium. we talked last time about decaying up the periodic table. what's gonna be the atomic number of the element that this will decay into? what number plus a negative one give me 92? 93. lee, okay. anyone else besides lee? how many of you say it's 93? all right. okay. you guys are just being a little reticent, right? okay. it's gonna be a 93. and that 93 is a brand-new element. it's beyond uranium called transuranic and that's neptunium. you know why they call it neptunium? you know what the first planet was they discovered in terms of newton's laws? neptune, gang. neptune. so they call this neptunium. first element beyond uranium and that's neptunium 239. it's got a very, very short half-life. and you know what it does? it decays. and guess what it emits? begins with a b. no, not alpha. try it again.
8:06 am
it emits a beta particle. okay. it emits a beta particle. and what does it turn into? how many people can figure out what the number is here that couldn't do it before but maybe now can? show of hands. four people. wonderful. now, how many? what is it gonna be, gang? it's 94. 94, 94. what is the second planet that we named after discovering by newton's laws? it wasn't neptune, it was what? - pluto. - pluto. guess what we'd be calling this, gang? plutonium. plutonium. pu239. and you know what plutonium will do? plutonium as well as uranium 235 will undergo fission when a neutron taps it. and so this is used for power production. u235, used for power production. historically, when the bombs were dropped back in world war ii, the bomb that devastated hiroshima was a uranium 235 bomb. you know how much uranium 235 was left after the bomb was detonated? almost none. the united states shot its arsenal, one bomb,
8:07 am
none left to speak of. you know what the bomb that hit nagasaki was? plutonium. plutonium. does the same thing. plutonium is made from uranium 235--238, very plentiful, but you got to bombard it with neutrons so you can make it. you can make plutonium. now, why do we get this 200 million electron volt? that's what i want you to know. you--all you guys know that uranium splits into elements and you get a lot of energy off, and you all know that the neutrons will cause new reactions. niceroony, that's junior high school knowledge, yeah, anyone takes a science course. what we wanna know is why all that energy, and i have a model for you to consider. and it has to do with mass. see on the table here, i have-- oh, it's imagination time. look, i have all the 92 elements in the periodic table that's found commonly in the earth's crust, almost all 92, are all arranged here. see the hydrogen here? see the hydrogen atom?
8:08 am
what's this one here? helium. helium. what's this one? lithium. lithium. i got no names, but you can just kinda look at them, right? what's the next one? i keep running after you. i know what the last one is. you guys know what the last one is, 92? begins with u. uranium. uranium. excellent. okay. and so i have all the atoms all lined up here. can you see them? at least in your mind's eye. now what i'm gonna do is i'm gonna shake the atoms. i'm gonna take the hydrogen-- just the nucleus of the atom, okay? i take the hydrogen, i shake it back and forth, okay? now, it's harder to shake the helium. how come? because it has more mass. there's four nucleons. over here is only one. then i take the lithium and i shake that, easier or harder? harder. what's the hardest one to shake? uranium. why? it got more mass. let's make a graph of how hard to shake versus atomic number. would that be fun? it'd be a drag. but let's do it anyway, okay? because i'm gonna lead it up to something that's kinda neat.
8:09 am
how hard is it to shake, that's mass, isn't it? mass, huh? inertia, yeah. versus atomic number. i'm gonna start out with one and i go to right up here to 92. all my atoms, yeah? my graph would look something like this, gang. it start to go straight. it turns out the first 20 or so atoms have as many neutrons as protons, so they kinda go straight up and then it starts to curve. and you know what this graph tells you? this graph tells you something not so wild, common sense. that if you wanna take the mass-- if you wanna know, find out how massive the uranium atom is, well, up here, we draw and there's the mass. this atom here, what mass it got? [whistling] you read the mass right there too, okay? this atom here-- [whistling] --what's got the lowest mass? hydrogen. and they keep building up more, more massive as they--huh, huh? nothing to it. big deal? no, it's no big deal. i'll show you a big deal. let's suppose we make a graph that's similar.
8:10 am
we make a graph of the mass per nucleon. that's different. mass per nucleon. what's that mean? that means this. i'm not gonna look like that, gang, i tell you that. you might think it looks something like this, which is to say, how much mass does a proton have? oh, it has just much mass here as here, as here, as here, as here, constant. true or false? begins with an f. false. falseroony, falseroony. let's go back to these atoms again. let's take the hydrogen. you shake it. now, go to hydrogen, then we go to helium and grab a nucleon. now, just grab one nucleon and shake it. ooh, easier. go to lithium, grab it. ooh, easier to shake, less mass for the protons
8:11 am
in here than in here. and the most mass here is, we'll find out. and you keep going up, up, up the periodic table and the nucleons are easier to shake. and you care to get up to iron, and it's very, very easy-- you got the least mass of all because beyond iron the nucleons seem to gain mass. and you're starting to come back up, when you get to uranium, you still don't have as much mass you had in hydrogen. what is going on? how massive a proton is, how massive a neutron is, apparently depends upon what configuration nucleus it's in, and that's the case. and yet you kinda see that with einstein's equivalence of mass and energy. consider you have a nucleus. you got all these nucleons in there, right? they're all being held together, yeah? very, very strongly, nuclear forces, yeah? now, you wanna take a nucleon, when i say nucleon, i'm talking about a proton or a neutron, okay? you take one out and you wanna take-- grab one and you wanna pull it out of the nucleus.
8:12 am
easy or hard? begin with the h. kinda big. there's nuclear forces, honey, and you're gonna pull against that nuclear force. it's gonna be hard to pull a nucleon out of that, gang. it's gonna be very hard to pull it out of there. you know what the hardest one to pull out is? an iron. you say to iron, "iron, honey, man, you got hardly any mass in your nucleons." iron says, "try pulling me out." [makes sounds] it's gonna take an awful lot of work to pull that nucleon out of iron. more out of iron than anything else. and when you pull these nucleons out, it takes work to do that. you need to work on that, like stretching a spring, you give it more and more what? begin with the e. energy. energy. and guess how that energy has manifest. begin with the m. mass. mass. and the nucleon will have more energy which is to say, equals-- which was easy to say will have more mass outside the nucleus than inside. and in there lies the secret of nuclear energy
8:13 am
because the graph of the nucleons would be something like this. over here 92, there's the graph that really, really tells it. and it's a graph, mass per nucleon versus atomic. now, it's not the mass of the whole atom, mass of the whole nucleus, it's the mass of each nucleon in the nucleus. and you say, in hydrogen it has the most mass of all. none of its mass is bound up with energy or binding anything else with it, it's all by itself. and as you continue to go up the periodic table, you get varying amounts of mass per nucleon. the point is, how much mass you got? depends per nucleon, depends what nucleus you're in. let's look at the uranium fission in terms of this relationship. here's uranium right here. the uranium atom has nucleons which have about this much mass each,
8:14 am
each, all right? what's gonna happen to the uranium when you bust it up into krypton and barium and those are halfway down the periodic table? that uranium nucleus is gonna end up one down here, one down here. and this atom here, let's suppose its barium-- [whistling] --that's got less mass. each nucleon there has less mass than you started with. you ever hear of this equation here? rest energy equals mass times speed of light square. okay? what happens to that mass you don't have anymore? i wonder what it-- where did it go? energy. energy. it's like you got uranium here, huh. you take the uranium, put it in the bathroom scale, boom, weight: 238. now take uranium and knock it in two pieces, boom, boom, now put the two pieces over here on one big bathroom scale, less than 238. when you bust that in half, the pieces don't add up to what--see, it's not like classically thinking. if you take a loaf of bread that weighs a pound and you bust it in two pieces, each piece is gonna be half a pound or the two pieces will add up to the pound, yeah?
8:15 am
that does not take place at the nuclear level. when you bust up one of these heavy elements, the pieces will have less mass than you started with. and that mass that's missing multiplied by the speed of light squared, and guess what you're gonna get for uranium, gang? 200 million electron volts, and there's your energy. you have converted mass, inertia to energy, and then there it is manifest in the kinetic energy of these particles, by part, as heat. you can boil water. you can boil that water, you can turn it to steam. you can pass that steam through a nozzle, you can put a paddle wheel at the other end, you can make a paddle wheel spin. and you know what you're going to do with that paddle wheel? you can hook it to a generator, and you make the generator spin. and you know what you can do? you have some wires coming in that generator, guess what flow through the wires? beginning with the e. electricity. electricity. e. only one big hassle, gang-- not only one big hassle but one big hassle about this is when you bust the uranium up, these two pieces here are neutron-rich. these are larger nuclide than their normal cousins. okay? there's extra neutrons, makes it bigger.
8:16 am
that's why i look at neutron, the nucleus is bigger and as you mentioned there, paul, more unstable and away you go. so these things are radioactive by-products, good or bad? oh, it depends. usually bad. what are you gonna do with them? okay. these are emitting radiations themselves. so that's a hassle and we're all familiar with the radioactive waste problem. but you know what, gang? there's a brighter side to the whole story of nuclear power. instead of talking about losing mass by going down-- this is like an energy hill, you read that in the textbook? think of it as an energy hill. you're rolling down the hill, you're picking up energy. that's what you're doing. you're kinda losing mass and that mass that you're losing so to speak, is what? goes off as energy. but here we have right in the middle, what's this element right here, gang, in the very, very bottom? iron. iron. and that's atomic number 26. how about over here? how about you taking an element like right about here? how about you taking element right in here? okay, i don't know what it is, maybe it's magnesium. and i take some magnesium and i split that too.
8:17 am
if i split that, what will happen? let's suppose i took an element here and i split it in two pieces, one here and one here. would i lose mass? or would i gain mass? let's try it. here i am here. i've to run through them over here. i got more mass than i started with. no energy off, gang. no energy off. the name of the game for any power production, if you're burning coal, is have less mass afterwards than you started. you got energy. if you got more mass afterwards than you started, you had to take in energy. but energy be given off if you got less mass afterwards than you begin. so in this side of the curve, on this side of iron, what do i have to do? break apart or do what? what's the other word? instead of break apart, what do i wanna do? fuse them. i wanna fuse them, honey. i wanna fuse them. i wanna take these isotopes here and i wanna fuse them together. let's suppose i take some high-- that's what the sun and the stars do. take some hydrogen, huh. hydrogen, look at all the mass it's got, yeah? now, take those hydrogen--
8:18 am
[makes sounds] --squash them together. easy or hard to do? hard. very hard to do, electrical repulsion. but if you could squash-- and sun and the stars do that, squash them together-- [makes sounds] --now, it's helium. helium got less mass per nucleon than the hydrogen had. now, don't get mixed up. helium has almost four times the mass as hydrogen, but not four times the mass per nucleon as hydrogen. hear what i'm saying? so it's mass per nucleon. you have to have that straight. otherwise, it doesn't make sense. so you got less mass per nucleon, hey, that mass per nucleon loss, boom, that's why the sun shines, that's why the stars shine, that's nuclear fusion. a typical reaction, for hydrogen, you're squashing-- it's typical fusion reaction. it happens in the sun and the stars, yeah? squash a couple of deuterium atoms together. and what you do is you get helium. helium is what? atomic number two, three, most helium is four, yeah? this one is three. where's the other one? right here. and guess what the energy that reaction has manifest?
8:19 am
the kinetic energy of these, mostly this. this thing go on up about a fifth the speed of light. watch out. don't get in the way of that. that thing pump anything, gonna heat them up. so you knew what you can do with a reaction like this too? you can put it in water, and guess what the water will turn into? steam. and the steams expands, right? and that expanding steam you direct right through a nozzle. guess what you put in the other side of nozzle. no, let me tell you, a paddle wheel. and a paddle wheel gonna spin right? and guess what you hook the paddle wheel to? generator. a generator. very good. the generator. and a generator is gonna generate what? electricity. yeah, you get electricity. so you can get electricity from either fission power or fusion power. and there's the fusing of elements, huh? another reaction is this. tritium. tritium. that's the heavy-- the heaviest isotope of hydrogen. and that goes to good ol' helium four plus the neutron. this is the stuff that you see in the kids' balloons, yeah? okay.
8:20 am
those reactions are a lot of energy. not so much energy per reaction as this. but you have a lot more those in a gram of matter than you do this. so gram per gram, you'll get more energy from nuclear fusion of little isotopes down on here than you will nuclear fission of heavy isotopes over here. the name of the game is, on this graph, gang, is to get a reaction that goes toward iron. if you're heavier than iron, you want something that after you are through, your products are closer toward iron. see, because you'd be losing mass. the name of the game, lose mass. on this side of iron, to go toward iron, you're gonna go this way. so what you do is you combine, you combine atomic numbers. you take things and you squash them together. and if they squash together, they lose mass, that mass that's lost-- they call it mass defects. they're not really lost, okay? that mass then is the energy of your reaction. and it really, really works out neat. if you understand all these, if you do,
8:21 am
you can answer this question. we said before the uranium will bust into krypton and barium. and when it does that, 200 million electron volts is the energy of the reactants, yeah? let's suppose instead, you took some krypton and you took some barium, a couple of nuclei, and you try to squash them together. it'll be very difficult. you know why? 'cause you got all these protons repelling, repelling, repelling. it'd be very, very difficult. but let's suppose you did do that and you fuse them together and snap. how much energy would it take to fuse them together so they snap? how many say, "i got no idea. this is nuclear physics. i'm not in the nukes." how many say, "well, when they flew w apart, "if they get 200 million electron volts, "maybe nature symmetric, maybe it'll take "200 million electron volts to put them back together again." that's it. that's it. you get it right back again.
8:22 am
because when you take these two things and slam them together, they'll have more mass afterwards. how much more-- that much more mass. and how much does that convert to energy watt? 200 million electron volts. so you see, when you try to fission things, i mean, when you try to fuse things on this side, if you fuse these things, you're gonna climb a hill. you're gonna gain mass. what you wanna do is lose mass after reaction. lose mass. if you understand that, you can answer this question. let's suppose you have some iron and with this iron-- it's in the future, we get fusion power plants, we get fission power plants. and in the future we have-- and you get some iron. you wanna bring the iron to the power plants so you can get some energy out of it. should you bring the iron to the fission power plant or the fusion power plant? check your neighbor. see if your neighbor be know it.
8:23 am
okay, gang. what be the answer? neither. what happen if you fission it? iron's 26. let's suppose your fission did exactly in half. then the two pieces would have what atomic number? 13. do you have to know what half of 26 is? 13. what is it? 13. is it 13? okay. it'd be two 13s, yeah? the two 13s-- [makes sounds] you'd have more mass afterwards. you ain't gonna get off-- you're not gonna get more mass and get more energy. come on, take your pick. so that won't work. let's suppose we take a couple of iron atoms when we fuse them. fuse them. now, it's gonna be, what? you'd have to know what 26 times 2 is. you got friends that could tell you, yeah? okay. you see it with--50-- 52. we can do it our self. hey, we can do these things our self, all right? okay? so be 52--52 is way up here. you have more-- [makes sounds] iron, honey, is the atomic sync, the nuclear sync. you'll get no energy out of iron. and when you start fusing atoms beyond iron,
8:24 am
you end up with mass being-- energy being absorbed. when you take your astronomy course, you're gonna learn about how the elements are made. they're made by hydrogen atom. you're gonna squash, squash, squash, fuse right up the periodic-- when you get up to iron, they start squashing them together, your star stops growing. your star starts to cool down, because when you start to fuse heavy elements like this, energy is required, not liberated. and your star then cools. all these elements here are made in catastrophic reactions when stars-- [makes sounds] --blow up. supernova. these elements are a lot more air than these elements down here. this is simple fusion, the sun and the stars, don't they? beyond there, takes different reactions all together. neat, how-- how we figure this all out. at least to this level. now, why haven't we figured it out yet? it keeps going, going, going. beautiful. now, it turns out the sun, the sun is burning, converting matter to energy every second.
8:25 am
every second go by 4 1/2 billion tons of matter are not there anymore. [makes sounds] that's sunshine. 4 1/2 billion-- every time you-- if you look at the sun today, you notice it's a little bit smaller than yesterday? 4 1/2 billion tons per second being sent off as radiation. like air. now, that sound scary? but the sun is what? begin with the b. big. end with g. got an i in middle. it is big, honey. it is a big, big sun. so big that at that rate, at the end of a million years, only 1/10 millionth of its mass is gone. so it's a very big sun. even bigger than us. bigger in this room, i mean, bigger-- bigger than the whole building, you know what i'm saying? it's a big, big--come on, i'm kidding. it's an enormous sun. but that's what the sun is doing. the sun is converting that to power. why don't we do that here on earth? why don't we take what the sun and the stars do and just do it here on earth and have fusion power. we got a lot of fission power plants.
8:26 am
how about fusion power plant? do you know how many fusion power plants there are in the world today? this is the 1990s, almost, yeah? about the 1990s. how many fusion power plants in the world? begin a z, honey, yeah. zero. no fusion power plants. hc. well, a century ago, there were no airplanes. people say, "how come there's no airplanes? "the birds do it. why don't we have airplanes? "why do we have to get our feet--from. "we go from one continent to another. "why don't you get in the great big airplane and go--sky?" what would people say a hundred years ago? our time will come. our time will come, but not yet. hmm? and now we say, "why don't we have fusion power plants?" you don't have any radioactive waste. this is a dream come true. you know, you get more energy out of 10 liters per water, per second, than you get out of all the power plants in north america, counting canada? 10 liters of water per second. i mean the poorest nation in the world can muster up 10 waters of liter-- 10 liters of water per second. that's sea water, any kind of water. if you could fuse the hydrogen in there, hmm,
8:27 am
you talk about big energy, gang. enormous energy. you're gonna--all this stuff about oil and whale oil. i mean, already we've forgotten about whale oil, right? when you guys-- you grew up burning whale oil? no--petroleum, right? what did your great grandfathers--whale oil. the world's changing. now, when we get up to, where we can harness this kind of thing. hmm--different, different. 'cause the fuel is the most abundant element in the universe hydrogen. over 90% of the universe estimated to be hydrogen. the universe, gang, as far as a human condition, is the same design for where we are now, where we would've been, or where we're going? it's all fusion fuel out there, gang. now, we haven't been able to do that now because it's very difficult to do. it was difficult to make airplanes fly at first too. interestingly enough, you know what the hang glider? the hang glider.
8:28 am
do you know when the-- you guys are too young to know about this. but the hang glider was developed after human beings got to the moon. i mean that one's kind of funny. yeah. the hang glider was developed by techniques at nasa working on moon projects. and after the human being got to the moon in 1969, then the advent of the hang glider came out. now, that's upside down. wouldn't you ever predict that differently if you are basically a futurist a hundred years ago? you know, just a kite, man. just get a kite, big enough, huh? so sometimes, the succession doesn't always go as you would expect. just before. say again? just before. oh, hang glider, just before? yeah, it's even designed to bring back the gemini. and they said it wouldn't work. but somebody said, "well, hey, what about the--" how soon before, paul? about three years--four years. let me correct that. you know, gang, the hang glider-- [laughing] thanks, paul. you know, paul casey is a science writer, you know, and he's taking our class? he writes--he makes his living writing science articles.
8:29 am
anyway, anyway, gang-- is this, gang, we have to be patient of where we are in our history. everything doesn't happen at once. we're still growing, you know? now, with this fusion, how come we don't have fusion power plants? it's too hard to take these things and push them together. it's enormous electrical repulsion. if you'd heat this stuff up to a gas, maybe above a hundred million degrees, these things will be fast-- going fast enough, they'll scrunch. how does the sun and the stars do it? brute force, gravity. [makes sound] i say, "i don't care electrical repulsion, no electrical repulsion." wham. right in. and the sun and the stars is just brute force these things together, and you get your fusion. now, how do we get that-- how do we do that in earth? we've been trying brute force. with plasma reactors. heat these things up to over a hundred million degrees, get that gas, pinch it with magnetic fields. boom. try to get it defused. has the human race done that? yes, we've done that. have we had a sustained reaction? no, we haven't had a sustained reaction. have we had any reaction to give off more energy than put in? no, we haven't. how come? our time has not yet come.
8:30 am
we're trying different techniques. we're trying it out with lasers. what we do is take little pellets. drop them. you had this in the textbook. little pellets of hydrogen. take all these lasers-- [makes sound] --wham. brute force smash these things together. and that should work too. sustained reaction, more energy out than required to operate the lasers? begin with an n. no. why? it's not our time. kind of a loose way of putting it. and there's another way, too. they've done it altogether without brute force. and i'm talking about cold fusion. i'm not talking about the utah experiments that caused all this publicity in 1989. i'm talking about--1988, was it? 1988? 18-- 1989, yeah. i'm not talking about those reactions, which, of course, were sort of the more publicity stunt than anything else. i'm talking about the cold fusion that's muon-induced. see, here's a problem with that. let's get into that just a little bit. let's suppose i got this proton over here,
8:31 am
and i want to this proton to fuse into that. well, once i get beyond the electron here, there's gonna be an enormous repulsion. very, very tough to do. okay? because this positive charges repel. but what if this electron were replaced by another particle that behave like an electron but was much, much more massive and would orbit way down in here. there is such a particle, and it's called a mu-meson. and a mu-meson has the same charge of an electron, and it will orbit very, very close. now, when this proton is coming over here, is it repelled by this configuration? no, it is not because it sees a neutral charge. it's very, very close. and what happens if it succeeded in doing this? spinning mu-mesons into this hydrogen gas, and it'll take the place of the electron. and this thing will come right over and, boom, snap, fuses at room temperature almost. and this is really, really neat. only one trouble. only one trouble. the mu-mesons are very, very short lived. they only last two billionths of a second.
8:32 am
but you know what? they can get--it turns out when this thing reacts-- [makes sound] --it fires out-- it fires the mu-meson back, and that can hit another one, and another, and another-- they have more like--more than a hundred times in its lifetime, a mu-meson could make a reaction. this is very, very exciting. this is cold fusion. and they do it--it works best at about 900 degrees fahrenheit. very, very cool compared to anything else. so read about this in the text and watch for it. it's kind of like a contender. there are problems because these mu-mesons are hard to come by. it takes energy to get those. and so far, we have-- we can't see a way that we can get more energy out of the reaction than it takes to fire it up to begin with. so these are problems that the technician-types are working on now. the physics-types and the technology-types are working on. but when--and if and when they're solved, things are gonna be like different. really different. questions? i didn't quite catch the deal on breeder reactors because,
8:33 am
say, losing mass is the name of the game. redirectors make it. let's go to that very, very quickly. what a breeder reactor does is very simple. if in your reactor you have 238 of plutonium-- or 235 of plutonium causing the fusion, if you put in some u-238-- i erased it over here-- the 238 will be turned to plutonium. so you're really not gaining any mass. what you're doing is you're converting something that's not very helpful, 238. you're converting that to something that is very helpful, energy-wise, plutonium. and the analogy i used in the book is like putting gasoline-- putting some water in your gasoline in your car. so it isn't like you get more mass than you started with. you're just converting the water to the gasoline. or in this case, you're converting the 238 to plutonium. and it turns out, every reactor is all the time breeding. if you have any 238 in there at all, it's gonna turn the plutonium. some of it's gonna do that. so all fusion reactors are, to some extent, breeder reactors.
8:34 am
it's just whether or not, you designed your reactors, so that's a main feature or not. but if we chose to make a reactor with plutonium, wasn't that the--on this table or that's-- what i remember from my reading is that there's always a chance that a plutonium reactor might explode. so that's somewhat-- to my knowledge, that's not the danger, no more dangerous with the plutonium than 235. it's not a question of exploding. if the chernobyl reactor didn't explode, it overheated. to cause these things to explode, to make a bomb, you have to contain-- you have to have it contained and crushed together. reactors won't explode. it won't blow up like a bomb. you wouldn't see like a hiroshima or a big cloud. no reactor will do that. what they'll do is they'll overheat. it will overheat and melt down. all this stuff will spew out and it gets into the environment.
8:35 am
is that good or bad? beginning with a b. yeah. but it's not an explosion. yeah. now, this thing over here, this is not even-- you can't-- this is at low temperature, and it's sort of self-sustaining. this has no chance at all of being used as a weapon, which is kinda-- this is all in the textbook. the neat thing is if we get to the point, we have like a fusion age. and all this idea of getting oil and things like that for fuel, we have no more problem with energy. and how do we make matter? matter is made by what? fusing things together. and i think the day will come when human begins will do that. what's the world gonna be like when there's abundant energy and abundant materials? no more scarcities? it's gonna be different. now is that gonna be good or it will be bad? it's hard to say. but i think of one thing, i think of my own story when i was a kid. i remember i used to-- i knew what i wanted to do. a lot of kids don't know what they wanna do when they grew up. i knew what i wanted to do. i had it all straighten there. i was gonna be an airplane pilot because i lived near--field. at--field, you see those guys there riding these little airplanes.
8:36 am
they put their caps on and the glasses and everything. put their cigarettes right up in here, okay? remember that? and they fly up there, man. we climb the trees and kinda look out. you know, you kinda see far, but these guys up above-- these airplanes. wheew. that's where it's at. i knew i was gonna be an airplane pilot, but i had a hang-up, like most kids do. and my hang-up was is that i didn't know how to tie my shoes. and my sister used to humiliate me 'cause i couldn't tie my shoes, and she could. and she was younger than me. and i was afraid when i get to be an airplane pilot that my sister was gonna tell on me. and all those people would humiliate me, and i would be humiliated. and i really had a problem about that. how am i gonna fly these-- well, i have to get the kind of shoes that you don't have to tie, you know? and i was really, as a kid, worried about that. and i think in a similar way, we kind of worry about what the future's gonna be too. not realizing by the time the future comes, honey, we'll be able to tie our shoes. we'll tie our shoes and do better than that. so when that future comes, we'll be ready for it. but a lot of changes, gang. think about these things.
81 Views
Uploaded by TV Archive on

 Live Music Archive
Live Music Archive Librivox Free Audio
Librivox Free Audio Metropolitan Museum
Metropolitan Museum Cleveland Museum of Art
Cleveland Museum of Art Internet Arcade
Internet Arcade Console Living Room
Console Living Room Books to Borrow
Books to Borrow Open Library
Open Library TV News
TV News Understanding 9/11
Understanding 9/11